 Port Credit Marine Survey & Yacht Delivery From Ontario, Canada Society of Accredited Marine Surveyors  American Boat & Yacht Council    
 |
AC DC ELECTROLYSIS MYTHOLOGY (as simple as
I can make it) ELECTROLYSIS SIMPLY CANNOT OCCUR ON BOATS ! In all forms of marine corrosion involving DC current, the anode is more negative than the cathode. In Electrolysis the exact opposite is true the anode is positive and the cathode is negative. Electrolysis is an induced process found in industry and laboratories and does not occur without designed intention. Electrolysis is used in many industrial applications such as electrowinning, electrorefining and electroplating but has absolutely nothing to do with boats. So do you really want corrosion or electrical advice from someone who does not understand the difference between stray current corrosion, galvanic corrosion and electrolysis ? What you are dealing with on corrosion on boats involving electricity is either "galvanic corrosion"or "stray current corrosion" which are electrochemical reactions that causes electrons to flow from one metal to another metal. One of the metals is the anode and the other is the cathode. If you put the two in an electrolyte that conducts current, and connect them with a wire, they act pretty much the same way a battery does. A current flows between the two metals. The electrons from one are "sacrificed" and plated (somewhat, but mostly dissipated) onto the other metal. This happens when you have dissimilar metals such as aluminum and bronze close to each other and in "electrical" contact , the aluminum corrodes. Punch line ........ Do not take advice from or hire a marine electrician who uses the term "electrolysis". |
|||
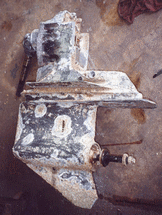
| 1. Galvanic corrosion : Corrosion that occurs at the anode of a galvanic
cell. There are four elements required before galvanic corrosion can occur. 1. an anode. 2.a cathode. 3. an electrolyte 4. and a current path between anode and cathode. You may remember high school chemistry class where a battery was created by connecting two dissimilar metals with wire and immersing the whole contraption in salted water thereby activating magnetic fields and starting an electrochemical process causing current to flow. On a boat with bronze, aluminum, galvanized and stainless steel that are connected with bonding wires or simply touching each other or mounted on the same metal ( zinc and bronze propeller on an ss shaft) and immersed in the lake/ocean ...... you accomplish the same thing. Each different metal has a different voltage potential. The more noble metal is the "cathode (+)", the less noble, the "anode (-)". In this process the less noble metal gives up electrons to the more noble thus weakening/wasting the anodic metal, otherwise known as "galvanic corrosion". The "sacrificial" anodes on your shafts, trim tabs etc. are supposed to sacrifice themselves thereby protecting expensive metal parts. This is why it's important to keep your anodes in good condition and never paint them. let's never refer to anodes as "zincs" as anodes come in three basic materials for different water conditions i.e. Aluminum alloy, and magnesium for fresh and brackish water or zinc for salt water. This topic deserves a little more attention on it's own so after this read Zincs, Aluminum and Magnesium Anodes. A vessel suffering from galvanic corrosion is usually the source of it's own problem, although two vessel's linked by shore power grounds can create a galvanic cell between two very close boats or perhaps a steel bulkhead. A Galvanic Isolator can protect your boat from DC current leaking into or out of your boat through the shower power ground. A GI cannot stop galvanic corrosion originating on your own boat. 2. Stray current corrosion (sometimes called electrolytic corrosion) : Corrosion that results from an electrical source causing a metal in contact with an electrolyte (water) to become anodic with respect to some other metal in the same electrolyte. In simple terms a wire touches something it shouldn't, like a faulty bilge pump float or degraded wiring lying in the bilge sending current into the water, causing one metal to give up electrons and corrode. Again any vessel suffering from this type of corrosion is likely the master of it's own disaster but the culprit could also be a neighboring vessel. This type of corrosion can can eat metals at an alarming rate. I know of one 42' motoryacht that lost both shafts, both rudders and both propellers in a space of less than two weeks. Complicating this picture somewhat is the
fact that DC can be super-imposed on your
AC wiring through the common ground on board
or the ground in the shore power pedestal
we all share on the dock. As all vessels
in the marina are connected through shorepower
grounds there is potential for widespread
damage. Aside from concerns of corrosion
there is also potential for electrocution
if shorepower cords are allowed to lie in
the water let alone the fools that leave
their shorepower cord plugged in at the dock
while they go out for an afternoon cruise
Common sources are faulty (or non-marine
battery chargers, inverters, bilge pumps,
some domestic electrical appliances or even
your water heater.. With our aging fleet of pleasure craft it's
likely that at some time, less than expert
hands have played with your electrical system.
If your vessel is suffering from any electrical
faults or unusual corrosion consult with
an American Boat and Yacht Council Certified
marine electrical technician with specific
corrosion control training or give me a call and I will try to set
you up with an expert in this field.
|
||||
| Wallace Gouk Society of Accredited Marine Surveyors AMS ret'd ABYC® Standards Certified ret'd ABYC® Certified Marine Corrosion ret'd ABYC® Certified Marine Electrical ret'd Transport Canada Licensed Master ret'd Transport Canada Tonnage Measurer BoatUS® Approved Marine Surveyor A Marine Surveyor for Ontario from Mississauga, Toronto, Hamilton to Oshawa, Whitby, Newcastle, Pickering, Trenton, Kingston, Gananoque and beyond |
||||